With the ongoing reforms in dental care and the widespread dissemination of oral hygiene knowledge, there has been a significant increase in public awareness regarding self-protection. Consequently, ensuring the sanitation of dental services has become a crucial issue of concern today. Dental equipment and instruments form the foundation and tools of oral medical diagnosis and treatment. Extensive research shows that these devices and instruments can act as vectors for healthcare-associated infections, which can be categorized into different transmission methods: surface contamination, airborne contamination, internal contamination, and direct injury transmission.
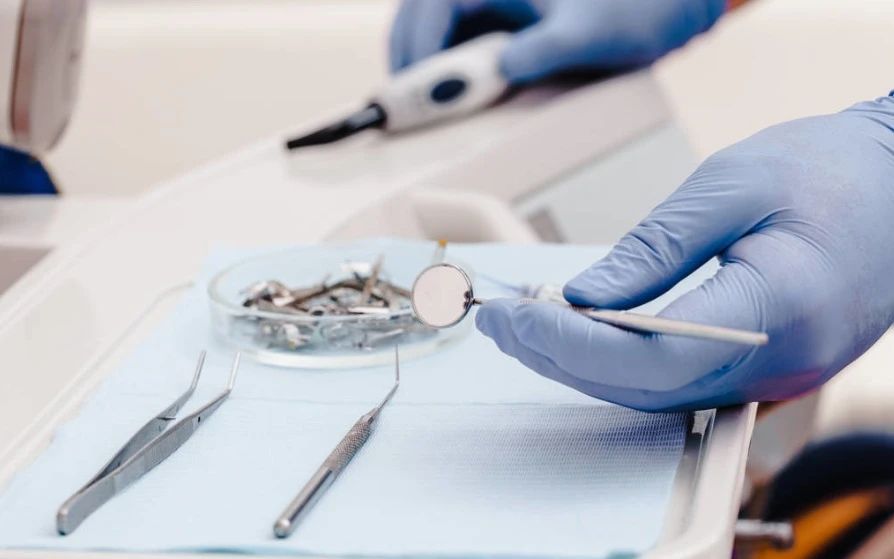
Dental procedures are predominantly performed inside the patient’s mouth, an organ that harbors the highest number of microorganisms. During treatment, dental instruments frequently come into contact with the patient’s blood, saliva, other secretions, and oral tissues. The risk associated with dental instruments can be classified into high, medium, and low categories. High-risk instruments include dental forceps, scalers, endodontic files, dental handpieces, and implant drills; medium-risk instruments include mirrors, orthodontic pliers, crown removers, and dental handpieces; low-risk instruments include model carvers, scalers, perforators, and dental hammers.
Currently, the rise in cross-infection rates within hospitals worldwide has attracted the serious attention of healthcare administrators and clinicians alike. Dental clinics must implement robust infection control protocols. Just as guests in luxury hotels expect their rooms, bathrooms, and public areas to be immaculate, patients too expect their dental care providers to adhere to top-notch infection control standards.
After disinfection or sterilization, instruments should not have the following issues:
- Residual materials from previous clinical treatments, such as adhesives, microorganisms, blood, or human tissues.
- Residuals from the cleaning process, including cleaning agents, disinfectants, or limescale.
- Contamination from dust, dirt, or environmental microorganisms during storage.
Cleaning and disinfection equipment and devices must adhere to these two fundamental principles:
- Instruments must be clean and sterile at the end of the cleaning process.
- Instruments must be maintained in a good clinical condition before use.
Technical Standards
In the UK and the EU, standardized manufacturing and operational guidelines for sterilizers and cleaning-disinfection machines are harmonized, such as BS EN ISO 15883 (for cleaning-disinfection machines) and BS EN ISO 13060 (for bench-top sterilizers). Many official UK clinical cleaning and disinfection guidelines incorporate these BS/EN standards to ensure that instrument cleaning and disinfection in dental clinics meet safety and effectiveness benchmarks (e.g., HTM 01-05, Scottish SHTM 2010, and SDCEP cleaning and disinfection practices).
Design of Dental Cleaning and Disinfection Units
In dedicated cleaning rooms, instruments are transported in a unidirectional flow, following a work pattern from contaminated to clean areas. A thermal washer-disinfector should be placed at the junction between the contaminated and clean zones. Ventilation should direct airflow opposite to the instrument transport direction, i.e., from clean to contaminated areas, to prevent aerosol cross-contamination. Specific operational recommendations include installing fans or exhaust systems on walls or windows; however, opening doors, windows, or movement within the room can obstruct airflow. Portable fans should not be used in cleaning areas, as they can cause air circulation pollution and disrupt the clean-to-contaminated airflow. The layout also includes guidance on personnel movement to ensure effective zoning. Dedicated cleaning rooms require high levels of discipline and training, making them more prone to human error compared to dual-room cleaning facilities.
In a dual-room cleaning facility, the first room is designated for washing contaminated instruments, while the second room is for disinfection, packaging, sterilization, and sterile storage of instruments. A double-door washer-disinfector is placed between the contaminated and clean rooms. The design may also include a manual transfer hatch as a restrictive entry point for passing contaminated instruments and equipment packages. Due to the low likelihood of cross-contamination between the two rooms, this design is preferred for cleaning areas.
Manual Cleaning of Instruments
- Dedicated Deep Sinks: Use sinks with separate compartments for washing and rinsing.
- Non-Foaming Cleaners: Avoid detergents that create foam, as foam can obscure sharp instruments.
- Temperature Measurement: Use a thermometer to measure water temperature or temperature-sensitive cleaners (water temperatures above 45°C can cause protein coagulation and hinder removal).
- Volume Measurement: Measure water and cleaner volumes to achieve the manufacturer’s recommended concentration. Graduated markings inside the sink can help indicate water depth. Add cleaner to water to minimize foam formation.
- Long-Handled Nylon Brushes: Use long-handled nylon brushes instead of cleaning pads or metal brushes. Sterile brushes (such as plastic polishing brushes) should be cleaned and sterilized after use or replaced with disposable items, and stored dry. Dental brushes should not be kept in disinfection baths and should be replaced regularly.
- Disassembly of Multi-Part Instruments: Disassemble multi-part instruments, paying special attention to gaps and connections.
- Rinsing: Rinse instruments in a separate rinse sink, avoiding splashes or aerosol creation. Keep instruments submerged during rinsing.
- Drying and Inspection: Use disposable non-woven cloths (or other disposable paper towels) for drying instruments. Inspect instruments under a magnifying light for damage or residue, and clean again if necessary. Instruments with rust should be discarded.
- Post-Cleaning Maintenance: After cleaning, drain sinks and clean sink surfaces. Instruments should be disinfected and sterilized as soon as possible to prevent corrosion and/or microbial regrowth.
- New Instruments: New dental instruments must be cleaned and sterilized before their first use unless pre-sterilized by the manufacturer.
Best Practices for Cleaning Dental Handpieces
- Thermal Washer-Disinfectors or Automatic Handpiece Sterilizers: Use these devices for cleaning, disinfecting, and sterilizing dental handpieces. If the manufacturer does not recommend thermal washer-disinfectors, consider handpiece cleaning equipment, which can only clean and lubricate, without disinfection or monitoring functions. Most modern dental handpieces (curved/straight/turbine) can undergo thermal cleaning and disinfection and are marked with safety symbols.
Recommendations for Using Disposable Dental Equipment
- Equipment with Narrow Lumen Difficult to Clean: Suction tubes and tips.
- Brushes: Demonstration toothbrushes, rubber polishing cups, and polishing brushes.
- Complex Instruments: Stainless steel handpieces, endodontic files/drills, impression trays.
- Invasive and Sharp Instruments: Forming plates, scalpels, needles, sutures.
Surface Cleaning Essentials
- Clean Items: Cabinets, operating room drawers, X-ray photos, patient records, computer keyboards, etc., should not come into contact with contaminated gloves or instruments.
- During Patient Treatment: Use waterproof drapes and plastic covers to simplify the cleaning process between treatments. Remove used covers and disinfect contaminated areas between treatments.
- End-of-Day Cleaning: Thoroughly clean and disinfect all surfaces in contaminated and clean areas. Replace and clean bend pipe filters regularly (preferably each night) and rinse thoroughly before replacement. Avoid using bleach or hypochlorite as they can cause rusting of filters. Follow manufacturer’s guidelines for replacements.
Ensuring Quality of Dental Unit Water Systems
- Backflow Prevention: Install backflow prevention valves on all water and gas delivery lines.
- Independent Water Supply: Use separate water supply systems for dental units.
- Water Quality: Fill storage bottles with freshly distilled or reverse osmosis water, not drinking water. Use antimicrobial agents (as per manufacturer’s instructions) in storage bottles and water lines. Daily, flush and disinfect the dental unit water system, storage bottles, and lines twice, preferably morning and evening.
- End-of-Day Procedures: At the end of the workday, disinfect storage bottles, rinse with fresh reverse osmosis or distilled water, drain thoroughly, and store inverted overnight.
- Pre-Flush: Pre-flush the dental unit water system for two minutes at the start and end of each day. Before treating the last patient of the day, pre-flush for 20-30 seconds. Replace or maintain antimicrobial filters daily or as per manufacturer’s instructions.
- Regular Flushing: Flush infrequently used dental unit water systems at least weekly. For invasive surgeries, use a separate sterile water system or saline to prevent backflow.